The world of precision manufacturing, from semiconductor fabrication to advanced aerospace coatings, the quality of a surface determines the success of the entire project.
Argon Ion Etching (AIE) has emerged as the gold standard for high-fidelity surface pre-treatment.
This article explores the technical nuances of optimizing Argon Ion Etching to ensure maximum adhesion, purity, and structural integrity of your substrates.
Argon Ion Etching is a dry physical etching process that uses ionized Argon gas ($Ar^+$) to remove surface contaminants and thin layers of material at the atomic level.
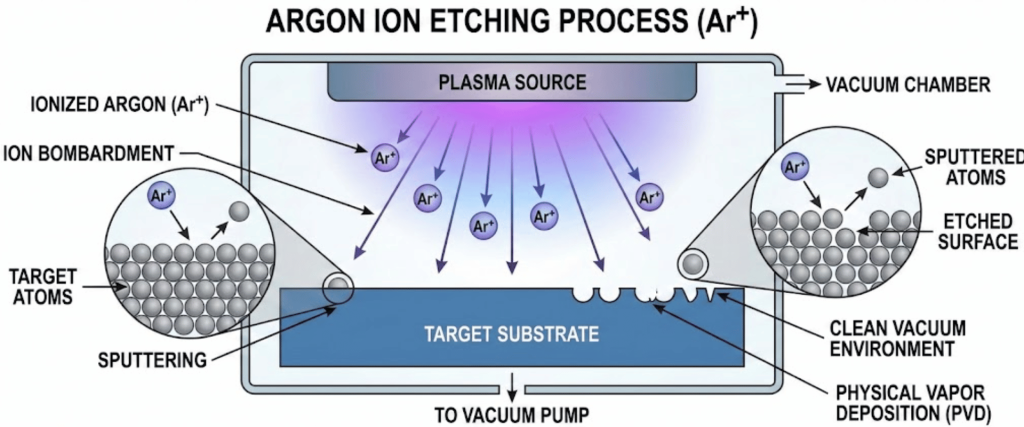
Unlike chemical etching, which relies on reactive substances, AIE uses kinetic energy to physically knock atoms off the target surface (a process known as sputtering).
Argon is the preferred medium because it is a noble gas. It is chemically inert, meaning it won’t react with the substrate or leave behind chemical residues.

Its relatively high atomic mass provides the punch needed to effectively clean hard surfaces like silicon wafers, metals, and ceramics.
Surface pre-treatment isn’t just about cleaning; it’s about surface activation. Improperly optimized etching can lead.
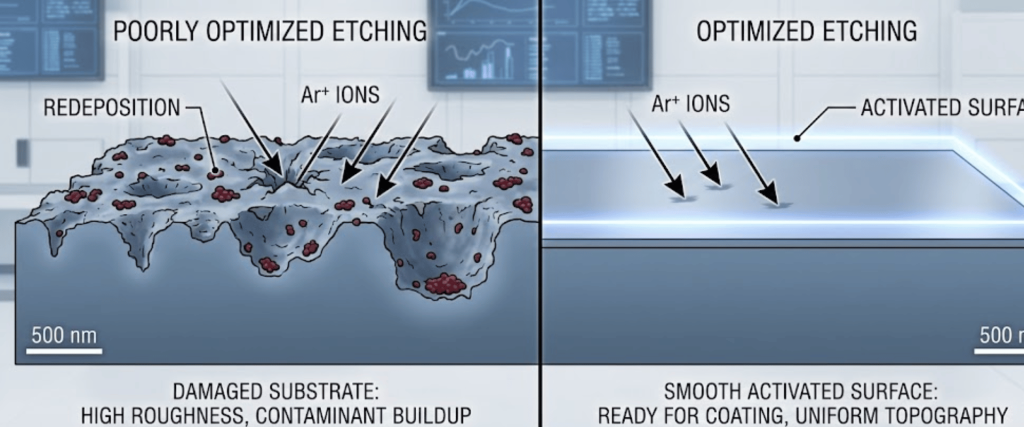
To achieve the perfect etch, several variables must be balanced. Here is how to fine-tune your Argon Ion Etching setup.
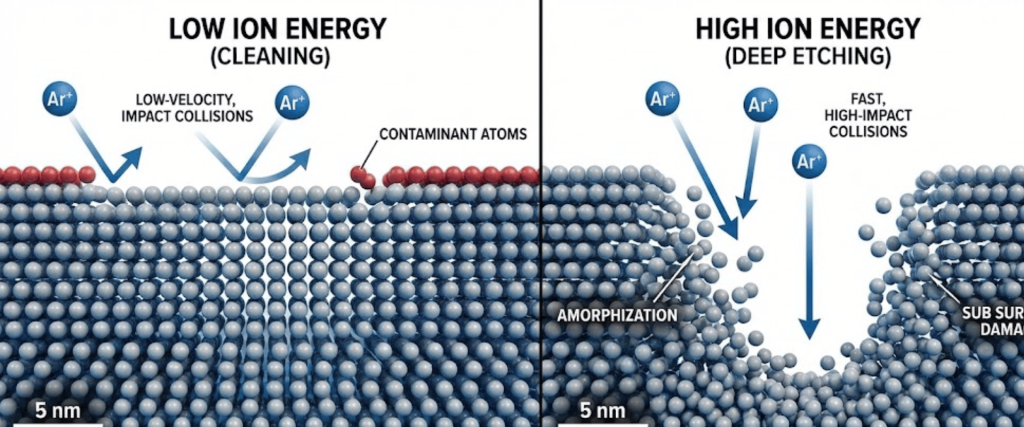
Ion energy determines the impact force of the Argon ions.
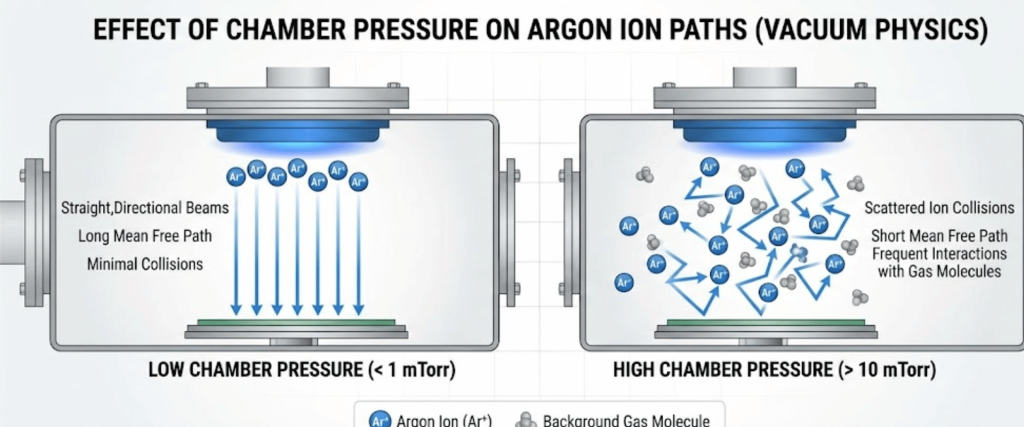
The pressure inside the vacuum chamber dictates the Mean Free Path of the ions.
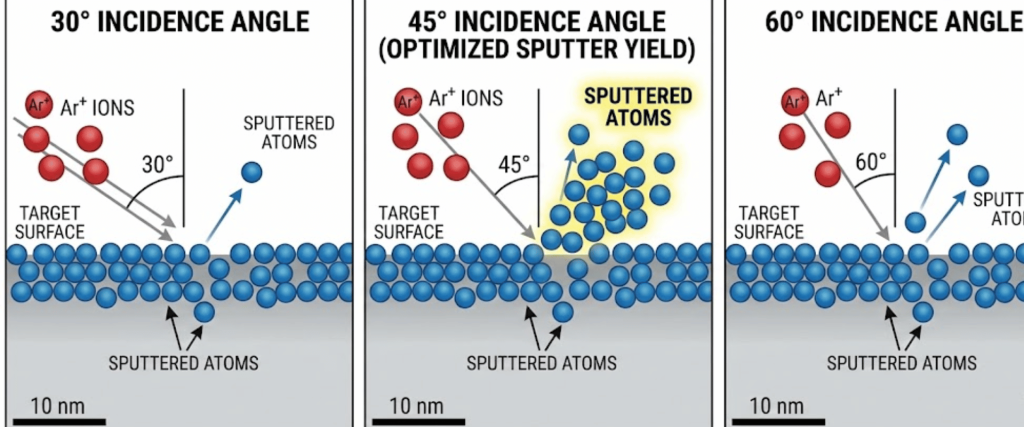
The angle at which the Argon ions hit the surface significantly affects the sputter yield.
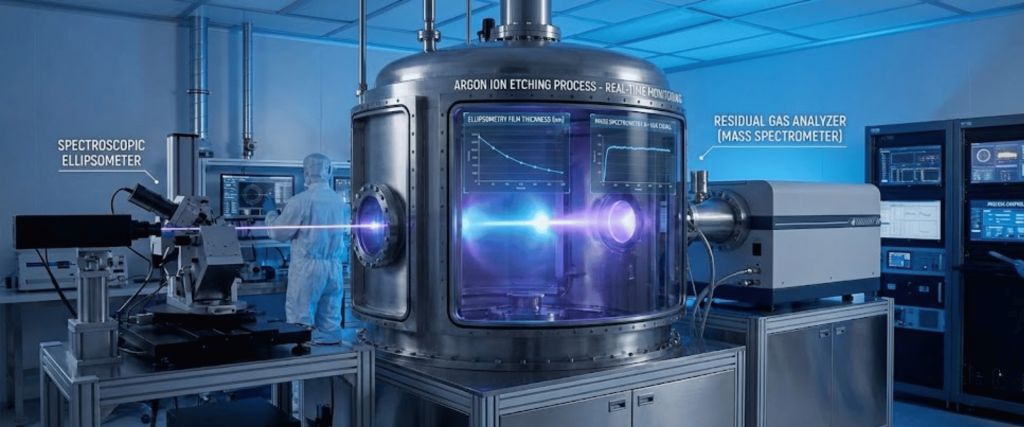
Over-etching is a common pitfall. Using In-Situ Monitoring (such as Ellipsometry or Mass Spectrometry) allows for real-time tracking of material removal to stop the process the moment the desired surface state is reached.
| Feature | Argon Ion Etching | Chemical Etching | Plasma Cleaning (O₂ / H₂) |
|---|---|---|---|
| Mechanism | Physical (sputtering) | Chemical reaction | Chemical + physical |
| Residue | None (inert) | Potential chemical traces | Minimal |
| Directionality | Highly anisotropic | Isotropic | Varies |
| Substrate safety | High (if optimized) | Risk of corrosion | High |

Optimizing Argon Ion Etching is a delicate balance between material removal and surface preservation.
By precisely controlling ion energy, pressure, and timing, manufacturers can create surfaces that are atomically clean and perfectly primed for the next stage of production.
Expert Insight: Always perform a post-etch surface analysis using XPS (X-ray Photoelectron Spectroscopy) to verify that all contaminants have been removed and that the stoichiometry of your surface remains intact.
Optimization is key to balancing material removal with surface integrity. By precisely tuning the Ion Energy (Bias Voltage) and the Angle of Incidence, the process ensures that only the top layers of contaminants or native oxides are removed. Using lower energy settings prevents the ions from penetrating too deeply into the crystal lattice, which avoids subsurface damage or lattice displacement, keeping the underlying material structurally sound.
Argon is the industry standard because it is a noble gas, meaning it is chemically inert and will not react with the substrate to create unwanted compounds. Its atomic weight is also ideal; it is heavy enough to provide the necessary kinetic energy to physically knock off surface atoms (sputtering), yet it is more cost-effective and easier to handle than heavier noble gases like Xenon. This results in an atomically clean, high-energy surface that is perfectly primed for bonding.
Since 1992, Applied Physics Corporation has been a leading global provider of precision contamination control and metrology standards. We specialize in airflow visualization, particle size standards, and cleanroom decontamination solutions for critical environments.