Nitride thin films, such as Titanium Nitride (TiN) and Chromium Nitride (CrN), are essential for extending the lifespan of industrial tools, but achieving maximum hardness requires precise control over the deposition environment.
The composition of the sputtering gas, specifically the ratio of inert Argon to reactive Nitrogen, dictates the stoichiometry, microstructure, and ultimate mechanical properties of the coating.
By fine-tuning these gas flow rates, engineers can manipulate the film’s grain size and density to achieve peak hardness values tailored to specific wear applications.
Mastering this balance is the key to transitioning from standard protective coatings to high-performance surface engineering solutions.
Reactive sputtering involves ejecting material from a metallic target (like Titanium or Chromium) in the presence of a reactive gas (Nitrogen) to form a compound film on the substrate.
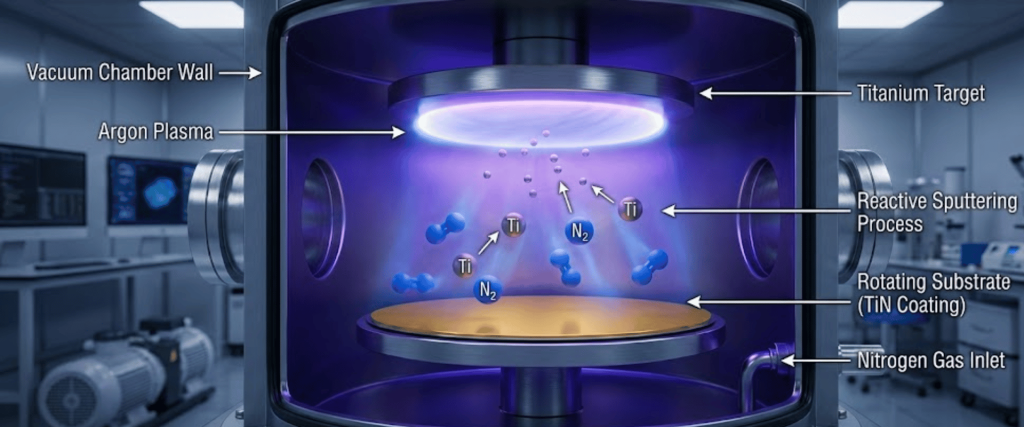
The gas composition in the vacuum chamber is the primary variable that determines whether you create a soft metallic layer or a hard, ceramic nitride coating.
Argon serves as the primary working gas. Being inert and heavy, Argon ions bombard the target material, physically ejecting metal atoms.
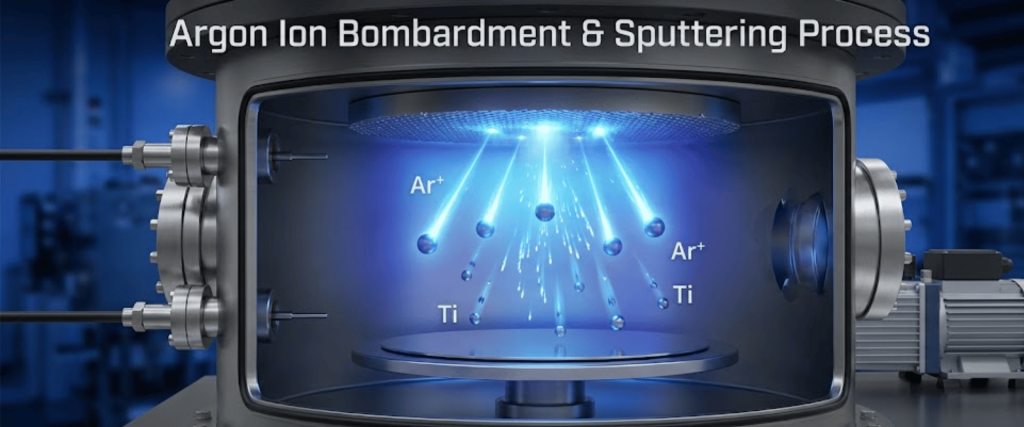
High Argon flow generally increases the deposition rate but does not contribute chemically to the film formation.
Nitrogen is the reactive component. It reacts with the ejected metal atoms (either in the gas phase or on the substrate surface) to form the nitride ceramic.
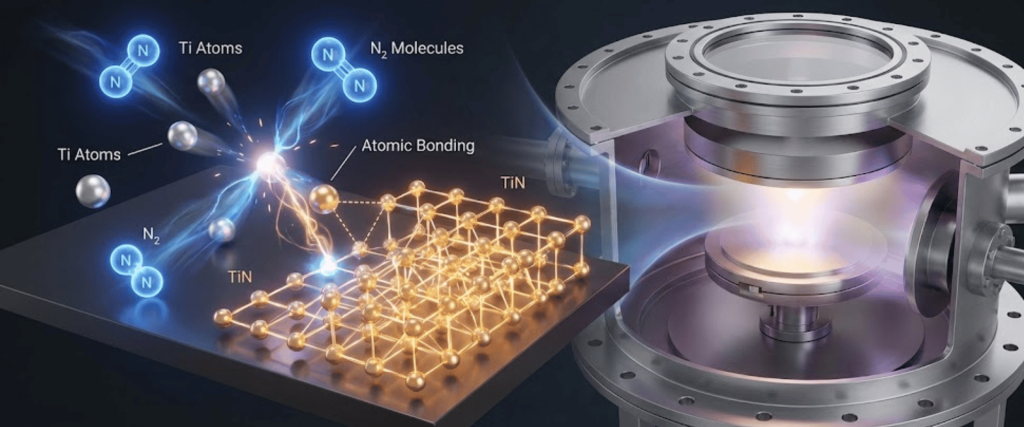
The partial pressure of Nitrogen is the most critical factor in defining the hardness of the film.
Optimizing hardness is not simply about adding more Nitrogen; it is about finding the stoichiometric limit where the metal and nitrogen atoms bond perfectly (e.g., Ti:N = 1:1).
If the Nitrogen flow is too low, the film remains under-stoichiometric. This means the coating will contain unreacted metal atoms.
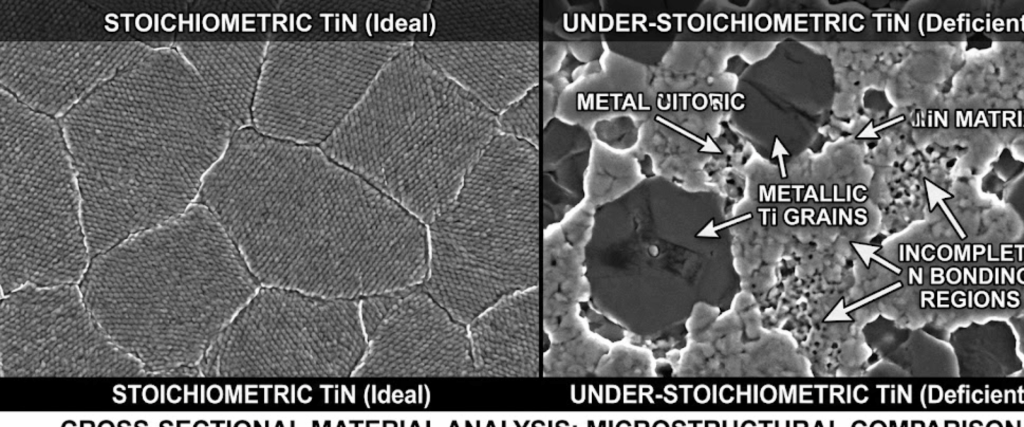
While these films may have better adhesion, they lack the ceramic hardness required for cutting tools and wear components. The resulting film often appears metallic and is softer than optimal.
Conversely, pushing the Nitrogen flow too high can lead to target poisoning. This occurs when the nitride compound forms on the target surface itself, rather than just the substrate.

Poisoning significantly drops the deposition rate and can alter the plasma dynamics, leading to defects in the film structure, which can actually reduce hardness and increase brittleness.
The gas composition directly influences the microstructure of the film how the atoms stack together.
One of the biggest challenges in optimizing sputtering gas is the Hysteresis Effect. As you increase Nitrogen flow, the process transitions from a Metallic Mode (high rate, low reaction) to a Poisoned Mode (low rate, fully reacted).
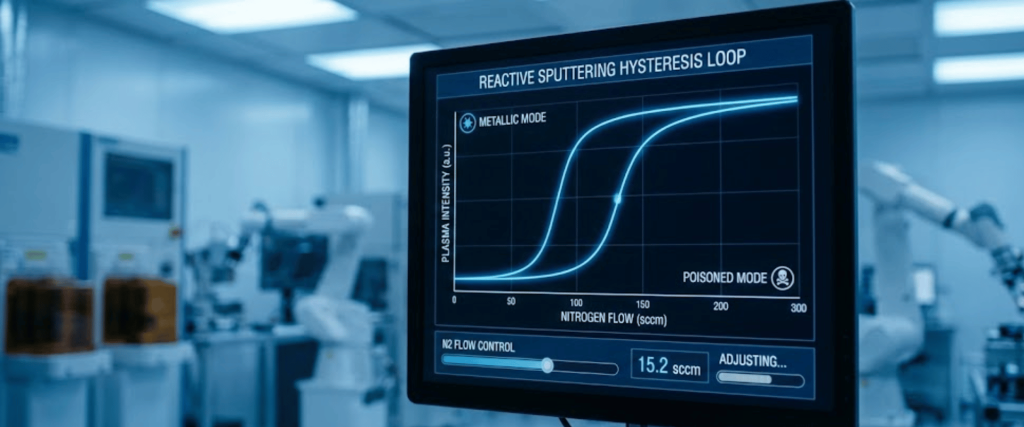
To achieve the highest hardness, the process often needs to run in the transition region a narrow instability zone between these two modes.
Optimizing sputtering gas composition is a balancing act between deposition rate and chemical reaction.
By carefully controlling the Argon-to-Nitrogen ratio and maintaining the process within the transition zone, manufacturers can produce nitride films with superior hardness, density, and wear resistance.
This optimization is critical for industries ranging from aerospace to medical device manufacturing, where component longevity is non-negotiable.
The ideal ratio varies by material, but maximum hardness is typically achieved in the transition zone. This is the specific point where there is just enough Nitrogen to form a perfect ceramic bond without over-saturating the process.
Too much Nitrogen leads to target poisoning, where the compound forms on the target surface itself. This drastically slows down the deposition rate and can introduce defects that make the film brittle rather than hard.
Argon is the working gas that physically ejects atoms from the metal target because it is heavy and inert. Without Argon, you wouldn’t have a stable plasma or a high enough deposition rate to build a thick, durable coating.
The industry standard for testing thin film hardness is Nano-indentation. This method presses a tiny diamond tip into the coating to measure its resistance to deformation without being affected by the softer substrate underneath.
Since 1992, Applied Physics Corporation has been a leading global provider of precision contamination control and metrology standards. We specialize in airflow visualization, particle size standards, and cleanroom decontamination solutions for critical environments.